
Jason Fontana, a molecular engineering Ph.D. student in the labs of chemical engineering professor James Carothers and chemistry professor Jesse Zalatan, has identified features of bacterial genes that impose strict requirements on CRISPR-Cas transcriptional activation tools. This work defines new strategies to effectively regulate gene expression in bacteria, bringing researchers closer to their goal of using bacteria to produce valuable biosynthetic products. Read this Q&A with Jesse Zalatan featured on the Science in Seattle blog.

A team was led by Dr. Alshakim Nelson, an assistant professor of chemistry at the UW, and Dr. Hal Alper, a professor of chemical engineering at the University of Texas, developed a new method that combines the bioactivity of microbes and a 3D-printed, synthetic hydrogel "” a water-based gel structure "” to create desired chemical compounds. The products can vary from pharmaceuticals to nutraceuticals, alluding to the vast potential for this new finding.
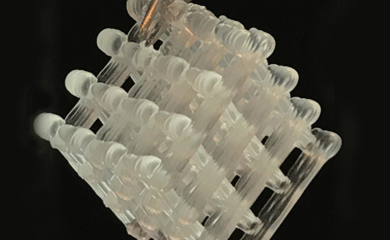
Researchers in the lab of MolES faculty member and professor of chemistry Al Nelson along with collaborators at the University of Texas unveiled a new way to produce medicines and chemicals and preserve them using portable "biofactories" that are embedded in water-based gels known as hydrogels. The approach could help people in remote villages or on military missions, where the absence of pharmacies, doctor's offices or even basic refrigeration makes it hard to access critical medicines and other small-molecule compounds.

An interdisciplinary research team led by MolES faculty member James Carothers, Dan Evans Career Development Associate Professor of Chemical Engineering, received a new $1 million research grant from the National Science Foundation (NSF) to investigate whether cells can learn.
In a paper published May 20 in the journal Nature Materials, a research team led by MolES faculty member Cole DeForest unveiled a new strategy to keep proteins intact and functional in synthetic biomaterials for tissue engineering. Their approach modifies proteins at a specific point so that they can be chemically tethered to the scaffold using light. Since the tether can also be cut by laser light, this method can create evolving patterns of signal proteins throughout a biomaterial scaffold to grow tissues made up of different types of cells.
Bioengineers have cleared a major hurdle on the path to 3D printing replacement organs with a breakthrough technique for bioprinting tissues. A research team led by MolES faculty member Kelly Stevens, assistant professor of bioengineering and investigator at the UW Medicine Institute for Stem Cell and Regenerative Medicine, has created exquisitely entangled vascular networks that mimic the body's natural passageways for blood, air, lymph and other vital fluids. The team published its findings May 3 in the journal Science. Their research was also featured in Newsweek, Forbes, among other outlets.
A team led by MolES faculty member and bioengineering Professor Valerie Daggett has developed synthetic peptides that target and inhibit the small, toxic protein aggregates that are thought to trigger Alzheimer's disease. Dylan Shea, a molecular engineering PhD student in the Daggett lab, was the lead author on a new paper describing these findings, published April 19 in the Proceedings of the National Academy of Sciences.
A research team at the University of Washington, including MolES faculty member Nathan Sniadecki, an associate professor in the Department of Mechanical Engineering, has created a novel system that can measure platelet function within two minutes and can help doctors determine which trauma patients might need a blood transfusion upon being admitted to a hospital. The team published its results March 13 in Nature Communications.
A recent publication from the Institute for Protein Design, located in the MolES building, describes a nanoparticle platform developed for a respiratory syncytial virus study that will also be applied to vaccine research on flu, HIV, and more. Seattle startup Icosavax will advance related clinical trials.
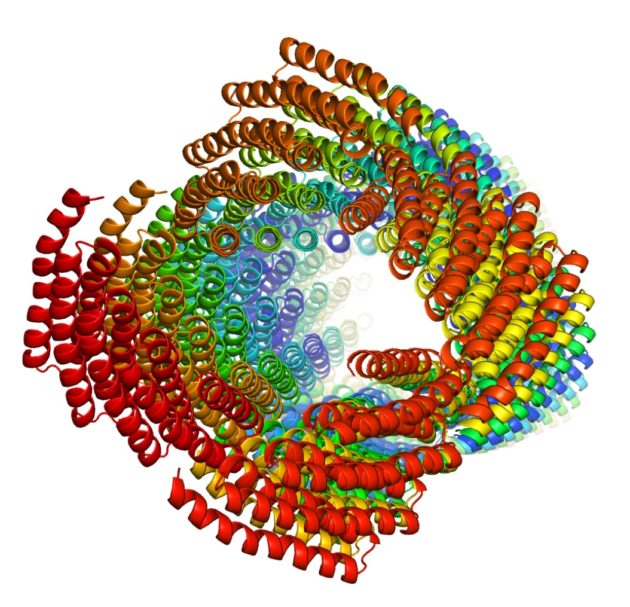
Hao Shen, a molecular engineering PhD candidate in the lab of biochemistry Professor David Baker, was a lead author of a study published in Science describing the creation of self-assembling protein filaments from scratch. The filaments were built from identical protein subunits that snap together spontaneously to form long, helical, thread-like structures which could be used to create new materials for a range of applications, from diagnostics to nano-electronics. Learn more in a related Geekwire story!